|
Leffingwell
& Associates
|
&
Molecular Structures
Several closely related GRAS chemical structure types are responsible for providing the "burnt sugar" type notes associated with many products (caramel, cotton candy, maple sugar, cooked fruits such as strawberry & pineapple, as well as roasted products such as chicory & coffee). Here we will examine the flavor properties (odor descriptions and intensities) and show the molecular structural similarities.
As will be noted, each of these very important flavor chemicals possesses the alpha-enol function adjacent to a carbonyl. Also of interest is that the replacement of methyl groups with ethyl groups in these structurally similar compounds generally results in an amplification of odor intensity.
Maltol or 3-hydroxy-2-methyl-4-pyrone...C7H8O3
.............................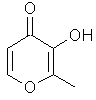
is an important flavor
chemical that occurs naturally in products such as
Caramel, Chicory, Cocoa, Coffee, Milk, Roasted Malt,
Strawberry, and Bread ... just to name a few. The odor is
often described as that of "cotton candy", the spun
caramelized sugar product sold at fairs.
Odor Detection Threshold (in water) = 35,000
ppb
Sweet, fruity, berry,
caramellic odor; fruity preserve-like. Very important in
commercial fruit flavors.
Ethyl
Maltol or
3-hydroxy-2-ethyl-4-pyrone...C8H10O3
..............................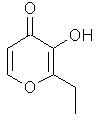
is the ethyl analog of
Maltol, wherein the 2-methyl group is now 2-ethyl. This
material is not occurring in nature. The odor is also
that of "cotton candy"; in fact the odor/flavor properties
are nearly identical except that Ethyl maltol is 4-5 times
as intense in flavor applications and perhaps slightly more
fruity. I have been unable to find a published value for its
odor detection threshold, but would expect it to be in the
range of 10,000 ppb.
Odor Detection Threshold (in water) =
NA
Sweet, fruity-caramellic odor;
fruity preserve taste
Furaneol(R) or 2,5-Dimethyl-4-hydroxy-3(2H)furanone ..C6H8O3
..........................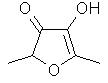
also known as "Strawberry
furanone" or "Pineapple furanone" was originally reported in
1965 by Willhalm, Stoll & Thomas at Firmenich & Cie
in Geneva as a key flavor component of strawberry
[Chemistry & Ind. [London], 1629,
(1965)]. At essentially the same time, Rodin, Himel,
Silverstein, Leeper & Gortner [J. Food Sci.,
30, 280 (1965)] found this material to be one of
the main organoleptic principles of
pineapple.
Today, we know that Furaneol
plays an important roll in the flavor of numerous fruits
(Chempedak Fruit, Guava, Litchi, Pineapple, Raspberry,
Arctic Bramble, Strawberry, Tomato and others), as well as
in roasted products such as coffee, corn tacos (maize),
cooked beef, malt, hazelnut, roasted almonds, and popcorn.
While the odor threshold has been reported by various
workers at values ranging from 0.4-1700 ppb, the value of 31
ppb seems most reliable. Buttery has shown that the
threshold value in water is pH dependant. Odor threshold in
water: 60 ppb at pH 7, 31 ppb at pH 4.5 and 21 ppb at pH 3.0
[Buttery, Ron G.; Takeoka, Gary R.; Ling, Louisa C., J.
Agric. Food Chem., 43(6), 1638-40 (1995)].
Recently, Yoshihiro Yaguchi, Atsufumi Nakahashi, Nobuaki
Miura, Daisuke Sugimoto, Kenji Monde, and Makoto Emura,
Stereochemical Study of Chiral Tautomeric Flavorous
Furanones by Vibrational Circular Dichroism, Org. Lett.,
2008, 10 (21), pp 4883–4885 and Makoto Emura, Yoshihiro
Yaguchi, Atsufumi Nakahashi, Daisuke Sugimoto, Nobuaki Miura
and Kenji Monde, Stereochemical Studies of Odorous
2-Substituted-3(2H)-furanones by Vibrational Circular
Dichroism, J. Agric. Food Chem., 2009, 57 (21), pp
9909–9915 have shown that the "strong, sugary, jammy,
sweet" character is due to the (+)-(2R)-Furaneol®.
Furaneol is a
trademark of
Firmenich.
Odor Detection Threshold (in water) = 31
ppb
Fruity, caramelized
pineapple-strawberry odor & taste
Furaneol
acetate or
2,5-Dimethyl-4-acetoxy-3(2H)furanone
..C8H10O4.
..........................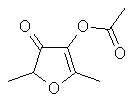
is the enol acetate of
Furaneol. This material was added to the FEMA (Flavor
extract Manufacturers Assn.) GRAS list in 1996. The author
has been unable to find a reference either to the occurrence
of this in nature or an odor detection threshold. However,
we find this material to possess better stability to air
than furaneol and believe it to have a superior cooked
strawberry note. It probably finds applications wherever
furaneol can be used.
Odor Detection Threshold (in water) =
NA
Cooked caramelic strawberry
note
Mesifurane
or 2,5-Dimethyl-4-methoxy-3(2H)furanone
..C7H10O3.
..........................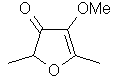
Although this material
accompanies the related furaneol
[2,5-dimethyl-4-hydroxy-3(2H)-furanone] (FEMA# 3174)
in Pineapple, Raspberry, Strawberry and Grape (and in fact
is higher in concentration in some cases, such as Arctic
Bamble) - and the material has a marginally lower odor
threshold. This material does not excite the flavorist as
much because its flavor is much more subtle and mellow. This
material is an excellent blender in Fruit flavors.
Sweet, fruity "sherry" like odor;
Xeres wine-like
note
Odor Detection Threshold (in water) = 0.3
ppb
Flavor threshold = 0.4 ppb (for the racemate)
Yoshihiro Yaguchi, Atsufumi Nakahashi, Nobuaki Miura, Daisuke Sugimoto, Kenji Monde, and Makoto Emura, Stereochemical Study of Chiral Tautomeric Flavorous Furanones by Vibrational Circular Dichroism, Org. Lett., 2008, 10 (21), pp 4883–4885 and Makoto Emura, Yoshihiro Yaguchi, Atsufumi Nakahashi, Daisuke Sugimoto, Nobuaki Miura and Kenji Monde, Stereochemical Studies of Odorous 2-Substituted-3(2H)-furanones by Vibrational Circular Dichroism, J. Agric. Food Chem., 2009, 57 (21), pp 9909–9915 have shown that (+)-(2R)-Mesifuran is "burnt, intensive caramel" and (-)-(2S)-Mesifuran has a "lactone, coumarin like, no caramelic odor".
Cyclotene
or
3-Methyl-2-cyclopenten-2-ol-l-one
..C6H8O2
..........................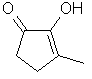
resembles furaneol and
maltol in that it has a 5-membered ring with its enol
carbonyl structure. Occurs in Cocoa, Coffee, Fenugreek,
Licorice, Malt, Roasted Almond and almost all products
containing sugar that are roasted. In conjunction with
fenugreek solid extract, "cyclotene" is an integral part of
nearly all Maple flavors. Several authors report the flavor
of this material to be somewhat similar to Licorice but, to
most North Americans, its flavor is closer to the syrup
obtained from a sugar maple.
Odor Detection Threshold (in water) = 300
ppb
Very strong, caramellic-maple,
lovage odor and taste
3-Ethyl-2-hydroxy-2-cyclopenten-1-one
or Ethyl
cyclotene
..C7H10O2
..........................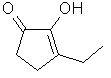
is the ethyl homolog of
cyclotene and possesses a similar odor and taste. Occurs in
Coffee, Tobacco & Tobacco smoke. Fenaroli (1975)
indicates the flavor threshold value for this material is "5
ppm" and that it also acts as a flavor enhancer. On a
relative basis to cyclotene, we expect that a more accurate
threshold value is about 150 ppb (however, this is
speculative, at this point).
Odor Detection Threshold (in water) = 5000
ppb
Very strong, caramellic-maple
odor and taste
3,5-Dimethyl-2-hydroxy-2-cyclopenten-1-one
or
Coronol(R)
..C7H10O2
..........................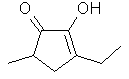
is 3,5-dimethyl analog of
cyclotene and possesses a similar odor and taste. Occurs in
Coffee & Tobacco smoke. Similar strong maple-caramel
notes. Coronol is a
trademark of
Givaudan-Roure.
Odor Detection Threshold (in water) = 1000
ppb
Strong, maple-caramel, nutty
odor/taste; similar to cyclotene
3,4-Dimethyl-2-hydroxy-2-cyclopenten-1-one
or Methyl
Corylone(R)
..C7H10O2
..........................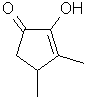
is the 3,4-dimethyl analog
of cyclotene and possesses a similar odor and taste. Occurs
in Coffee, Tobacco Smoke & Wood Smoke. Similar strong
maple, burnt-sugar, caramel odor/taste. This material is the
most potent of the cyclotene analogs.
Methyl Corylone is a
trademark of
Givaudan-Roure.
Odor Detection Threshold (in water) = 17-20
ppb
Strong, maple-caramel, nutty
odor/taste; similar to cyclotene
3-Ethyl-2-hydroxy-4-methylcyclopent-2-en-1-one
..C8H12O2
..........................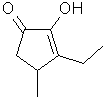
is the ethyl homolog of
3,4-Dimethyl-2-hydroxy-2-cyclopenten-1-one
and has a somewhat more burnt,
caramellic, maple-like odor. To the authors knowledge this
material has only been reported in Tobacco smoke. Similar
strong maple-caramel
notes.
Odor Detection Threshold (in water) =
NA
Strong, burnt, caramellic,
maple-like odor
3-Ethyl-2-hydroxy-5-methylcyclopent-2-en-1-one
..C8H12O2
..........................
is the ethyl homolog of
3,5-Dimethyl-2-hydroxy-2-cyclopenten-1-one
and has a more burnt, caramellic,
maple-like odor. To the authors knowledge this material has
only been reported in Tobacco & Tobacco smoke. Similar
Burnt, caramellic, maple odor as the 3-ethyl-4-methyl
isomer.
Odor Detection Threshold (in water) =
NA
Strong, burnt, caramellic,
maple odor
Sotolon
or
4,5-Dimethyl-3-hydroxy-2(5H)-furanone..C6H8O3
..........................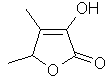
Sotolon is the key
organoleptic principal of roasted Fenugreek seed. Also found
in Roasted Virginia Tobacco, Sake, Sugar, Botrytized grape
wine and Rice wine. Until the materials shown on this page
became available, Fenugreek and Fenugreek extracts were the
only good flavor concentrate source for caramel-maple notes.
A product known for many years under the trade name of
"Mapeline" was used by housewives and industry whenever this
type of flavor was needed. Perfumers valued the powerful
Fenugreek absolute for such notes. The taste and aroma of
brown sugar is mainly due to sotolon (which is the main
flavor principal in sugar molasses). However, Sotolon is now
available commercially and is one of two extremely powerful
aroma chemicals for imparting caramel-maple
notes.
Sotolon with a detection
threshold of 0.001 ppb is nearly 30,000 time more powerful
than cyclotene. The chemical which follows is even more
powerful.
Sotolon is also known as caramel furanone, sugar lactone and
fenugreek lactone - it has the typical aroma of fenugreek or
curry at high concentrations and caramel, maple syrup and
burnt sugar at lower concentrations.
(+)-(S)-Sotolone is "curry, walnut
(strongly caramelic)" while (-)-(R)-Sotolone is "walnut,
rancid". Although the aromatic nuances of both enantiomers
were quite similar, the perception threshold of (S)-sotolon
in dilute alcohol solution (12% vol) was >100 times lower
than that of (R)-sotolon. See Pons, A.; Lavigne, V.;
Landais, Y.; Darriet, P.; Dubourdieu, D., Distribution and
Organoleptic Impact of Sotolon Enantiomers in Dry White
Wines, J. Agric. Food Chem.; (Article); 2008; 56(5);
1606-1610; Guichard, E.; Fournier, N. Enantiomeric ratios of
sotolon in different media and sensory differentiation of
the pure enantiomers, In European Food Chemistry VI;
Hambourg, 1991.
Odor Detection Threshold (in water) = 0.001
ppb
The odor thresholds in 12%
EtOH/Water V/V for the enantiomers
are:
(+)-(S)-Sotolone - Odor
threshold = 0.8
ppb
(-)-(R)-Sotolone - Odor
threshold = 89 ppb
Maple
furanone or
5-Ethyl-3-hydroxy-4-methyl-2(5H)-furanone..C7H10O3
..........................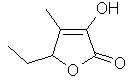
is truly one of the most
outstanding of modern flavor materials. This material is
much more powerful and lasting than Methyl or
Ethylcyclopentenolone and possesses more of a maple note
than Sotolon. Maple furanone is
a key organoleptic note in Soy sauce (hydrolyzed vegetable
protein). With a detection threshold of 0.00001 ppb maple
furanone is nearly 3,000,000 times more powerful than
cyclotene and in fact is one of the most powerful flavor
chemicals known to man.
Odor Detection Threshold (in water) = 0.00001
ppb
Powerful maple-caramel aroma
and taste
Data abstracted from
Flavor-Base
Copyright John
C. Leffingwell
1999-2010
Leffingwell
& Associates
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY