|
|
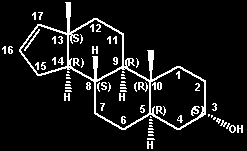
ent-(-)-5-alpha-androst-16-en-3-alpha-ol
- Much weaker with a detection threshold level
about six orders of magnitude higher than
(+)-5-alpha-androst-16-en-3-alpha-ol
=
(3S,5R,8S,9R,10R,13S,14R)-androst-16-en-3-ol
=
(3S,5R,8S,9R,10R,13S,14R)-2,3,4,5,6,7,8,9,10,11,12,13,14,15-tetradecahydro-10,13-dimethyl-1H-cyclopenta[a]phenanthren-3-ol
Ref: G.
Ohloff, B. Maurer, B. Winter & W. Giersch,
Structural and Configurational Dependence of the
Sensory Process in Steroids, Helv. Chim. Acta,
66, 192-217 (1983); G. Ohloff, Scent &
Fragrances, Springer-Verlag (1994), p.
27
|
|
|
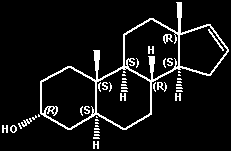
(+)-5-alpha-androst-16-en-3-alpha-ol
- Animalic, musk-like odor with a strong (fine)
sandalwood tonality
Threshold:
0.02 ppb (in air)
=
(3R,5S,8R,9S,10S,13R,14S)-androst-16-en-3-ol
=
(3R,5S,8R,9S,10S,13R,14S)-2,3,4,5,6,7,8,9,10,11,12,13,14,15-tetradecahydro-10,13-dimethyl-1H-cyclopenta[a]phenanthren-3-ol
Ref: G.
Ohloff, B. Maurer, B. Winter & W. Giersch,
Structural and Configurational Dependence of the
Sensory Process in Steroids, Helv. Chim. Acta,
66, 192-217 (1983); G. Ohloff, Scent &
Fragrances, Springer-Verlag (1994), p. 27;
Bajgrowicz, J.A. & G. Frater (2000), Chiral
recognition of sandalwood odorants, Enantiomer,
5, 225-234
|
|



