|
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com Leffingwell & Associates
|
Beverage-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The world's leading program for beverage development. Juice-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The leading program for development of juice containing beverages. ****** Menthol Background ****** Laevo- or (-)-menthol is one of the most important flavoring chemicals. Menthol is used extensively in pharmaceuticals, cosmetics, toothpastes, chewing gum, and other toilet goods as well as in cigarettes. The current annual world production is estimated to be in excess of 19,000 metric tons. The majority of (-)-menthol is obtained by freezing the oil of Mentha arvensis to crystallize the menthol present. This "natural" menthol is then physically separated by centrifuging the supernatant liquid (called dementholized cornmint oil) away from the menthol crystals. Any residual impurities are due to traces of Mentha arvensis oil, and these often impart a slight peppermint aroma to the menthol crystals. Considerable effort has been devoted to the production of (-)-menthol by synthetic or seini-synthetic means from other more readily available raw materials. Historically, the price of naturally derived (-)-menthol has fluctuated widely. This situation is now buffered to a degree by the two major comercial synthetic sources of (-)-menthol (Symrise and Takasago). However as the production of menthol in India continues to soar --- and raw material costs continue to increase for the synthetic producers, it appears that India will continue to increase market share with increasing margin pressure on Symrise & Takasago. In 2007, Clark4 estimated the world production of natural menthol at 12,870 metric tons and synthetic menthol at 6,300 metric tons for a total of 19,170 metric tons. Although, the exact production in each area are rough estimates, the producing exporting sources are estimated to be:
In 2007, worldwide consumption of (-)-menthol, by product area, was estimated to be4:
In 1998, Clark5 estimated the world production of menthol at 11,800 metric tons. Although, the exact production in each area are rough estimates, the producing exporting sources are estimated to be:
In 1992, the major producing exporting sources were estimated to be:
Figures on this page are adapted from information in references 2 & 3 found below. 1. Leffingwell, J.C. & R.E. Shackelford, Laevo-Menthol - Syntheses and organoleptic properties, Cosmetics and Perfumery, 89(6), 69-89, 1974 2. Hopp, R., Menthol: its origins, chemistry, physiology and toxicological properties, Rec. Adv. Tobacco Science, Vol. 19, 3-46 (1993). 3. Enberger, R. and R. Hopp, Synthesis and sensory charachteristics on menthol enantiomers and their derivitives for the use of nature-identical peppermint oils., Topics in Flavor Research, R.G. Berger, S. Nitz and P. Schrier, Editors, H. Eichorn, Marzling, Germany, pp. 201-218 (1988). 4. Clark, G.S., Aroma Chemical Profile: Menthol, Perfumer & Flavorist, Vol. 32 (12), 38-47 (2007). 5. Clark, G.S., Menthol, Perfumer & Flavorist, Vol. 23 (5), 33-46 (1998).
|
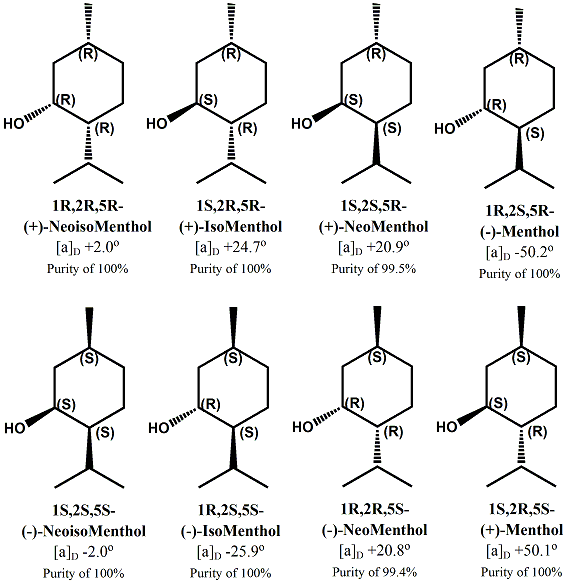
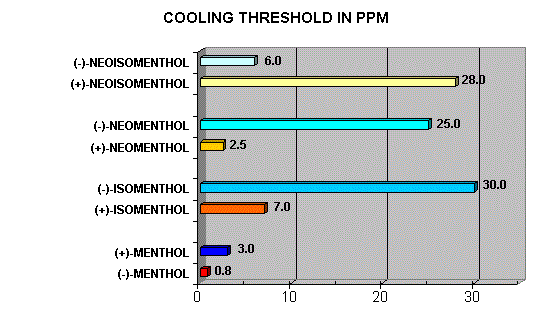
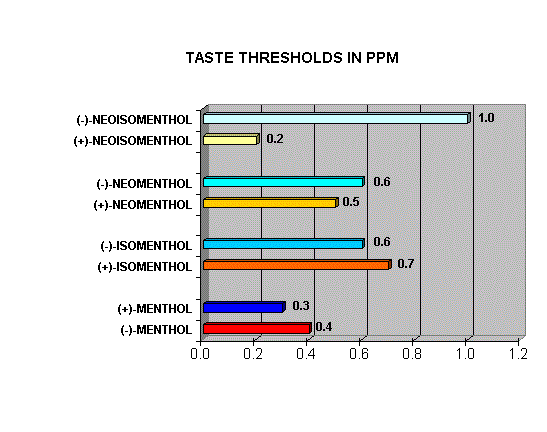
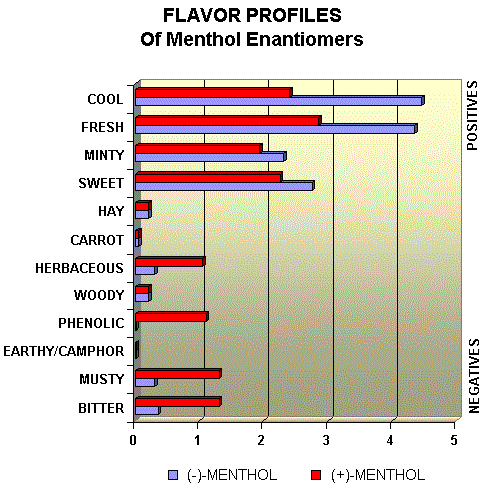
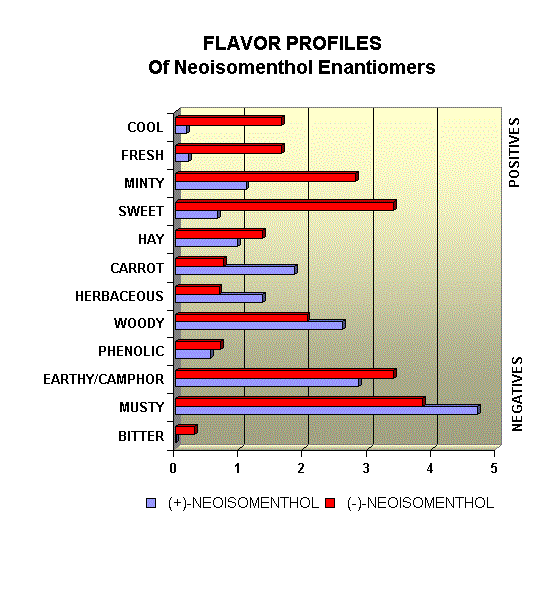
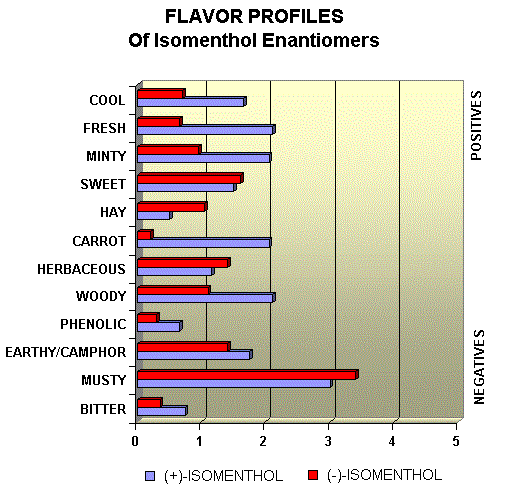
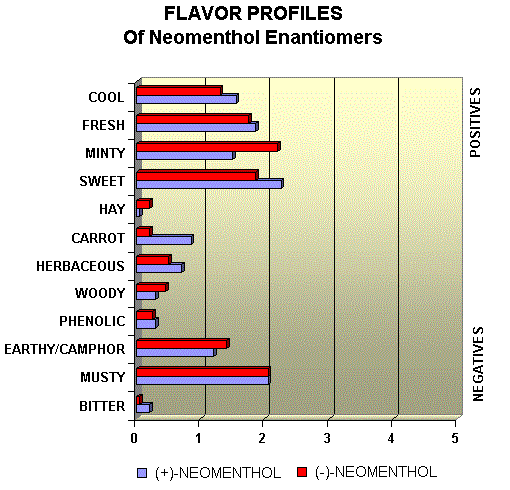
Menthol Enantiomers - Organoleptic Properties
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY