|
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com Leffingwell & Associates
|
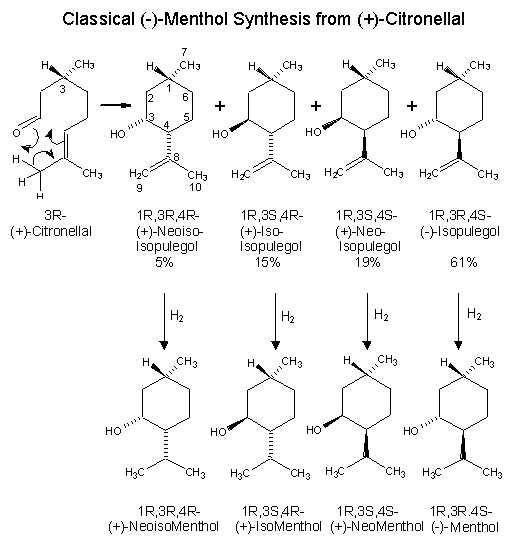
Beverage-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The world's leading program for beverage development. Juice-Master 2011 - NEW - A new version with enhanced features for Excel 2007 & 2010. The leading program for development of juice containing beverages. The production of (-)-menthol from citronella oil is probably the most popularized synthetic of the procedures, since it is used as an illustration in many organic chemical textbooks. This process has been used commercially both in the United States and abroad whenever the price ratio of citronella oil to natural menthol was advantageous. (+)-Citronellal is separated from citronella oil by fractional distillation and then subjected to cyclization procedures. Classically, this cyclization is carried out in protonic media (formic acid, phosphoric acid, acetic anhydride, or solid catalysts such as silica). Ohloff has shown that thermal cyclization occurs acidic cleanly and in high yield.3 The resultant isopulegol isomers formed possess the necessary asymmetric center at C-1 of the p-menthane skeleton for (-)-menthol production. The (-)-isopulegol can be physically separated and directly hydrogenated to (-)-menthol, while the remaining isopulegol isomers are recycled to an equilibrium mixture.3 Alternatively, the total isomeric mixture of isopulegols can be hydrogenated to an isomeric menthol mixture. (-)-Menthol is separated and purified through high efficiency distillation columns and recrystallization (of derivatives). The remaining isomeric menthols can be isomerized catalytically to an equilibrium mixture favoring the desired (-)-menthol isomer. This process of physical separation of (-)-menthol or (-)-isopulegol and isomer equilibration can be continued at will and is an integral part of the commercial production of (-)-menthol from (+)-citronellal. The retention of optical activity in this process is determined completely by the fact that the initial asymmetric ceriter in (+)-citronellal is carried through the entire sequence unchanged. Configurations at C-3 and C-4 in the resultant p-menthane skeleton are manipulated to always correctly relate to C-1. Maintenance of such a center of spatial configuration is mandatory for any successful (-)-menthol synthesis from optically active starting materials. This process can be optimized by using zinc bromide or a tris(2,6-diarylphenoxy)aluminum catalysts as cyclization catalysts for converting citronellal to isopulegol. Zinc bromide provides a ratio of (-)-isopulegol to other isopulegol isomers in the ratio of 94 : 6, while tris(2,6-diarylphenoxy)aluminum catalysts can give a ratio of (-)-isopulegol to other isopulegol isomers in the ratio of 99.7 : 0.3. See reference 4 which you can download. 1. Leffingwell, J.C. & R.E. Shackelford, Laevo-Menthol - Syntheses and organoleptic properties, Cosmetics and Perfumery, 89(6), 69-89, 1974 2. Hopp, R., Menthol: its origins, chemistry, physiology and toxicological properties, Rec. Adv. Tobacco Science, Vol. 19, 3-46 (1993). 3. Ohloff, G. Tetrahedron Letters, 10 (1960); R.L. Webb, U.S. Patent No. 3,218,361 (1965) 4. HORI YOJI; IWATA TAKESHI; OKEDA YOSHIKI, Process for producing isopulegol , European Patent No. EP1225163 (July 24, 2002) assigned to Takasago International Corporation
|
|
|
|
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY
