|
Leffingwell &
Associates
|
Alchemist WebPick Awarded by the webzine of ChemWeb.com  |
|
General Physiology of Olfaction Trigeminal Sense in the Olfactory Epithelium The Odorant Binding Proteins Odorant receptors The Cellular Membrane G-Protein Coupled Receptors G-Proteins The cAMP Transduction Cascade Ion Protein Channels Other Second Messengers in Olfaction - cGMP, IP3, NO, CO Chemical Olfactory Stimulation - Theories on Olfaction The Steric Theory of Odor The Vibrational Theory of Odor Vibrational Induced Electron Tunneling Spectroscope Theory Ribonucleotides as the Odorant carrier? Recent Events in Olfactory Understanding A Combinatorial Process for odor Interpretation Combinatorial Process Visualization Human Olfactory Receptor Genes Enantiomeric Specificity in the Olfactory bulb |
Olfaction-Page 4 John C. Leffingwell, Ph.D. Chemical Olfactory Stimulation - Theories on Olfaction Over the years a number of theories relating odorant quality to molecular structure have been proposed. Here we will review the two most prominent theories and add another involving the direct participation of certain neurotransmitters or their hydrolysates in assisting the docking of odorant molecules with the olfactory receptor protein. The Steric Theory of Odor In 1946, future Nobel laureate, Linus Pauling26 indicated that a specific odor quality is due to the molecular shape and size of the chemical. Similarly, in the book, "Molecular Basis of Odor" by John Amoore27, he extended the idea of a "Steric Theory of Odor" originally proposed by R.W. Moncrieff in 194928 that stated air borne chemical molecules are smelled when they fit into certain complimentary receptor sites on the olfactory nervous system. This "lock and key" approach was an extension from enzyme kinetics. Amoore proposed primary odors (ethereal, camphoraceous, musky, floral, minty, pungent and putrid). The molecular volume and shape similarity of various odor chemicals were compared (by making hand prepared molecular models and physically measuring volume and creating silhouette patterns - there were no computer molecular modeling programs in that era). The steric theory is well suited to the idea that the odorant receptor proteins accept only certain odorants at a specific receptor sites. The receptor is then activated (by conformation deformation?) and couples to the G-protein and the signal transduction cascade begins. The Vibrational Theory of Odor In 1938, Dyson29 suggested that the infrared resonance (IR) which is a measurement of a molecules vibration might be associated with odor. This idea was popularized by R.H. Wright in the mid 1950’s as infrared spectrophotometers became generally available for such spectral measurements which Wright was able to correlate with certain odorants.30 During the 60’s and early 70’s, vigorous debate raged as to the validity of each theory for classifying chemical odorants. By the mid-70’s, it appeared that Wright’s theory had failed a critical test. At the June 1970 "International Symposium on Gustation and Olfaction", Leffingwell and others challenged Wright's theory as the optical enantiomers of Menthol31 and of Carvone,32,32 smelled distinctly different, although the corresponding infrared spectra were identical. And this theory fell from favor. Leffingwell has now published on the internet an extensive site that provides over 710 enantiomeric pairs of odorants that often (but not always) have differing odor properties. This site provides both 2-D and 3-D molecular structures along with odor descriptors, odor thresholds and original references. Vibrational Induced Electron Tunneling Spectroscope Theory Until the seminal dissertation of Luca Turin33 in 1996, the vibrational theory had been placed under a very dark cloud. Turin, however, has attempted to provide a detailed and plausible mechanism for the biological transduction of molecular vibrations that, while not accepting the mechanical vibrational spectroscopy theory previously proposed, replaces it with a theory that the receptor proteins act as a "biological spectroscope". What was proposed is a process called "inelastic electron tunneling". Since this paper, which appeared in Chemical Senses in 1996 is available for downloading off the Internet [at http://chemse.oxfordjournals.org/content/21/6/773.full.pdf ], I will only outline the process of electron transfer proposed. Suffice it to say that the receptor is triggered by an odorant in the presence of NADPH (ß-Nicotinamide Adenine Dinucleotide Phosphate, Reduced Form), which is formed by the enzymatic reduction of ß-Nicotinamide Adenine Dinucleotide Phosphate (NADP). NADPH is widely distributed in living matter and acts as an enzyme cofactor. ß-NADPH is a product of the pentose phosphate pathway, a multifunctional pathway whose primary purpose is to generate reducing power in the form of ß-NADPH. ß-NADPH transfers H+ and 2e- to oxidized precursors in the reduction reactions of biosynthesis. Thus, ß-NADPH cycles between catabolic and biosynthetic reactions and serves as the carrier of reducing power in the same way that ATP serves as the carrier of energy.34 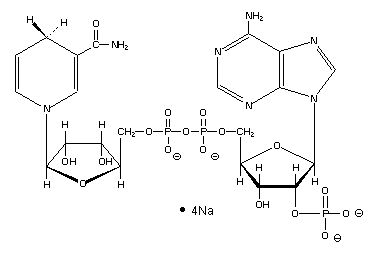
NADPH (as Sodium salt)
Since according to Turin’s theory the receptor functions as an "NADPH diaphorase", it may be significant that high levels of diaphorase activity have been detected in olfactory receptor neurons.35 In order for such electron transfer to occur, Turin proposes from molecular modeling that a zinc binding site is present both on the odorant receptor protein and the G-protein. Zinc’s involvement in olfaction, its ability to form bridges between proteins, its presence in electron transfer enzymes, such as alcohol dehydrogenase and the presence of a the redox-active amino acid cysteine in the receptor’s zinc-binding motif all point to a possible link between electron flow and G-protein transduction. "Suppose the zinc-binding motif on the olfactory receptor is involved in docking to the olfactory G-protein g(olf), and that the docking involves formation of a disulfide bridge between receptor and G-protein. One would expect to find on g(olf) the other half of a zinc coordination site, for example two histidines in close proximity, and a cysteine nearby." A search in the primary sequence of g(olf) finds the motif (His-Tyr-Cys-Tyr-Pro-His). This motif has the requisite properties for docking. It is exposed on the surface of the G-protein and is known to interact with G-protein coupled receptors. In the closely-related adrenergic receptors, a role for cyclic reduction and oxidation of disulfide bridges has been suggested (Kuhl, 1985)36 . It involves cross-linking of the G-protein to the receptor by an S-S bridge which is then reduced upon binding of the (redox-active) catecholamine to the receptor, thereby releasing the G-protein. Turin proposes that a similar mechanism may be at work in olfaction. Electron tunneling basically is the transfer of electrons down the backbone of the protein and here this would only occur as follows: "When the (olfactory) receptor binding site is empty, electrons are unable to tunnel across the binding site because no empty levels are available at the appropriate energy. The disulfide bridge between the receptor and its associated G-protein remains in the oxidized state. When an odorant (here represented as an elastic dipole) occupies the binding site, electrons can lose energy during tunneling by exciting its vibrational mode. This only happens if the energy of the vibrational mode equals the energy gap between the filled and empty levels. Electrons then flow through the protein and reduce the disulfide bridge via a zinc ion, thus releasing the G-protein for further transduction steps. If there is a molecule between the electron source and electron sink, and if that molecule vibrates then (taking the energy of the vibrational quantum as E) indirect tunneling can occur by an additional channel if there is an energy level in the source with energy E above that in the sink. After tunneling, the molecule will have a vibrational energy higher by E. In other words, tunneling occurs only when a molecular vibrational energy E matches the energy difference between the energy level of the donor and the energy level of the acceptor. The receptor then operates as a spectrometer which allows it to detect a single well-defined energy, E . If the change in energy between donor and acceptor levels is sufficiently large, tunneling current flows across the device only when a molecule with the appropriate vibrational energy is present in the gap. If there are several vibrational modes, which one(s) get excited will depend on the relative strengths of the coupling, and that may be expected to depend, among other things on the partial charges on the atoms and the relative orientation of the charge movements with respect to the electron tunneling path."33 Turin’s theory at the time seemed plausible, but from the beginning had numerous of critics. However, even if generally valid, it does not necessarily mean that the "Steric or shape Theory" doesn’t play a role. In the 20+ years since Turin's initial proposal was publicized on BBC television in 199533a, a rather uncivil discourse between Turin and critics has ensued. In 2003, Avery Gilbert scathingly reviewed Chandler Burr's book "Emporor of Scent"33b (in which Burr, in an openly partisan manner, recounts Turin's battle with the scientific community to accept his theory). Gilbert's quote of "In a grand substitution of ego for psychophysics, Turin claims that Turin’s theory successfully predicts odors because they smell the way Turin says they do"33c was generally accepted by much of the scientific community. In 2004, Nature Neuroscience published an editorial entitled "Testing a radical theory" that introduced a paper by Andreas Keller & Leslie B Vosshall entitled "A psychophysical test of the vibration theory of olfaction"33d. After testing a variety of psychophysical predictions of vibration theory, as formulated by Turin, they concluded that molecular vibrations alone cannot explain the perceived smell of an odorous molecule. This has prompted a even further series of papers by both critics and supporters of the vibration theory. We wish to mention that in 2006, Charles Sell published an article entitled "On the Unpredictability of Odor" which reviewed the difficulties chemists face in predicting the relationship between molecular structure and odor. As he noted, "Surprises are still commonplace, and serendipity continues to be an important factor in the discovery of novel fragrant molecules".33e Among some of the more recent attempts to clarify support for the validity of Turin's vibration theory are those of Jennifer Brookes, et al.33f-33k, Solov'yov et al.33l-33m, Turin et.al.33n-33s, while the critics include include Block33t, Vosshall33u & Sell.33v In 2008, Sell & coworkers published an article "Odorant–receptor interactions and odor percept: a chemical perspective" which appears to be the most reasonable of all in that it predicts that theories like "shape" or "vibration" are far to simplistic to predict odor. They state "From a biological perspective, a rather different picture emerges. First, Polak's33w prediction of a combinatorial mechanism was confirmed by Malnic et al.33x. Thus, it is now known that each odorant activates a range of receptor types, and each receptor type responds to a range of odorants." But Turin remains convinced he is right, no matter the conflicting facts. In the July 2016 issue of Perfumer & Flavorist, he discusses his theory with Pia Long. Another publication on olfaction we recommend is the article by Sophie Barwich entitled "What is so special about smell? Olfaction as a model system in neurobiology"33y. [The so-called electron tunneling concept in proteins is a major topic of interest as to the exact mechanism of electron transfer. This stems largely from the work of Jacqueline Barton (Electron transfer between metal complexes bound to DNA: is DNA a wire?) at the California Institute of Technology.37,37a ] Ribonucleotides as the Odorant carrier? It is now obvious, perhaps, that a multiplicity of events occur in olfaction. But several major questions that were not addressed earlier have now been answered.. 1. Are certain neurotransmitters (or their hydrolysates) involved not just as so-called "second messengers" in the transduction cascade, but are they also involved as "Amplifiers" that help to capture the odorant molecules and direct them to the receptor sites? The answer is YES! As early as the mid 1980's it began to become clear to Lancet's group in Israel that certain ribonucleotides increase in concentration and in adenylate cyclase activity.37b And from a practical medical standpoint, Henkin, et al.37c have shown that by increasing the concentration of cAMP and cGMP in human nasal mucus for patients with anosmia led to improvement in smell function. While there are a few intriguing clues in the literature relative to these questions, it appears that the potentially powerful electrostatic affinity properties of some of these neurotransmitters (or their hydrolysates) may possibly play a significant role early in the olfactory process. In the chemoreception of "taste", it has long been known that certain ribonucleotides (especially 5’-guanosine monophosphate [5’-GMP] and 5’-inosine monophosphate [5’-IMP]) have potent synergistic effects with MSG (monosodium glutamate)38 including a significant lowering of the MSG threshold level. In 1980, Torii and Kagan showed that a several-fold enhancement of binding of glutamate occurred with bovine taste papillae in the presence of certain 5'-ribonucleotides (e.g., 5'-GMP, 5'-IMP) but not with others (e.g., 5’AMP).39 Now it should be noted that 5’-IMP and 5’-GMP (as their sodium salts) commercially are used extensively as flavor enhancers, especially for meat and fish products to enhance meaty, brothy and the "uamani" character (often in conjunction with MSG to take advantage of the synergistic flavor enhancement). It has also been observed that there is a large synergism was observed between MSG and two species of nucleotides (GMP and IMP) in most mongrel dogs40 and between MSG and three species of nucleotides (GMP, IMP, and AMP) in beagles. This has also been observed for GMP and glutamate in mice.41 Chemically, it should also be noted that 5’-inosine monophosphate is the product of enzymatic deamination of 5’-adenosine monophosphate. For example, In meat extracts, after slaughter, there is as rapid transformation of 5’-ATP to 5’-AMP to 5’-IMP. In olfaction, very few studies are available that show activity of these ribonucleotides in enhancing olfaction. However, Getchell has demonstrated that 8-Bromo-cAMP applied to the ciliated side of the mucosa of the bullfrog caused a concentration-dependent, reversible increase in the basal short-circuit current, but not when it was applied to the submucosal side. Pulses of 8-Bromo-cAMP and odorant presented simultaneously resulted in currents that added nonlinearly.42 In addition, 5'AMP odorant binding sites on the dendrites of the olfactory receptor neurons in the sensilla of the spiny lobster are distributed along the entire dendritic region that is exposed to odorants. The distribution of these 5'AMP binding sites is considered much more extensive than that of enzymes that inactivate 5’-AMP.43 The few implications of ribonucleotides to playing an active part in what I will refer to as the extracellular side if the receptor neurons in the mucosa has largely been overlooked probably due to two factors: (1.) the ribonucleotides are largely water soluble and have not been examined as possibly complexing with lipid odorants and (2.) researchers have focused on the intracellular second messenger activity of such compounds. Results in our laboratory using molecular modeling and molecular fitting programs, however, show that 5’AMP, 5’cAMP, 5’GMP, 5’cGMP and IP3 all demonstrate dramatic electrostatic affinity for fitting with many odorants. For example, when compared to fitting with 5’-ATP, 5’-ADP, 5’-GTP or 5’GDP, the [computed] electrostatic fitting energy is of an order of magnitude 105 - 106 more favored. _______________ 26. Pauling, L., Molecular architecture and Biological Reactions, Chem. Eng. News, 24, 1375 (1946); referenced by Ohloff, G., Scent and Fragrances, Springer-Verlag, Berlin Heidelberg, 1994 27. Amoore, J.E., Molecular Basis of Odor, C.C. Thomas, Pub., Springfield (1970); Amoore, J.E., Johnston Jr, J.W. and Rubin, M., The stereochemical theory of odor. Scientific American, 1964, 42-49.; Amoore, John E. "Current status of the steric theory of odor." Annals of the New York Academy of Sciences 116, no. 2 (1964): 457-476.; Amoore, John E. "Specific anosmia and the concept of primary odors." Chemical Senses 2, no. 3 (1977): 267-281.; J.E Amoore, Pelosi, P. and Forrester, L.J., 1977. Specific anosmias to 5alpha-androst-16-en-3-one and omega-pentadecalactone: the urinous and musky primary odors. Chemical Senses, 2(4), pp.401-425. 28. Moncrieff, R.W., What is Odor. A New Theory, Am. Perfumer, 54: 453 (1949); Moncrieff, R.W., 1954. The characterization of odours. The Journal of physiology, 125(3), p.453. 29. Dyson, G.M., The Scientific Basis of Odor, Chem. Ind., 57: 647-651 (1938) 30. Wright, R.H., The Sense of Smell, CRC Press, Boca Raton, FL (1982) 31. Leffingwell, J.C., in Gustation and Olfaction, G. Ohloff and A. Thomas, Ed., Academic Press, NY, 1971, p. 144; also challenged by M.G.J Beets; G.Schneider; A. Dravnieks31a. 31a. Gustation and Olfaction, G. Ohloff and A. Thomas, Ed., Academic Press, NY, 1971, p. 145. 32. Langenau, E.E., Olfaction and Taste, Vol. III., C. Pfaffman, Ed., Rockefeller University Press, New York (1967); although this fact had been known to perfumers and flavorists 50 years earlier. 33. Turin, L., A spectroscopic mechanism for primary olfactory reception. Chem. Senses, 21, 773-791 (1996); Turin, L., 1997. The nose as spectroscopist. Chemistry and industry, (21), pp.866-870. 33a. BBC Horizon - A Code in the Nose (1995) - TV series
available at - Part 1 (https://www.youtube.com/watch?v=h25b3FrFV-A); 33b. Burr, Chandler, The Emporer of Scent - A Story of Perfume, Obsession, and the Last Mystery of the Senses, Random House, New York, 2002, ISBN 0-375-50797-3 33c. Gilbert, A.N., The Emperor's new theory, Nature Neuroscience 6, 335 (2003) 33d. Keller, A. & L.B. Vosshall, A psychophysical test of the vibration theory of olfaction, Nature Neuroscience 7, 337 - 338 (2004) 33e. Sell, C.S., 2006. On the unpredictability of odor. Angewandte Chemie International Edition, 45(38), pp.6254-6261. 33f. Brookes, J.C., Hartoutsiou, F., Horsfield, A.P. and Stoneham, A.M., 2007. Could humans recognize odor by phonon assisted tunneling?. Physical review letters, 98(3), p.038101. 33g. Brookes, J.C., Horsfield, A.P. and Stoneham, A.M., 2009. Odour character differences for enantiomers correlate with molecular flexibility. Journal of The Royal Society Interface, 6(30), pp.75-86.. 33h. Brookes, J.C., 2009. A microscopic model of signal transduction mechanisms: olfaction (Doctoral dissertation, UCL (University College London)). 33i. Brookes, J.C., 2010. Science is perception: what can our sense of smell tell us about ourselves and the world around us?. Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, 368(1924), pp.3491-3502. 33j. Brookes, J.C., Horsfield, A.P. and Stoneham, A.M., 2012. The swipe card model of odorant recognition. Sensors, 12(11), pp.15709-15749. 33k. Brookes, J.C., 2011. Olfaction: the physics of how smell works?. Contemporary Physics, 52(5), pp.385-402. 33l. Solov'yov, I.A., Chang, P.Y. and Schulten, K., 2012. Vibrationally assisted electron transfer mechanism of olfaction: myth or reality?. Physical Chemistry Chemical Physics, 14(40), pp.13861-13871. 33m. Reese, A., List, N.H., Kongsted, J. and Solov'yov, I.A., 2016. How Far Does a Receptor Influence Vibrational Properties of an Odorant?. PloS one, 11(3), p.e0152345. 1-21 33n. Turin L.,, Yoshii F., (2003) Structure-odor relations: A modern perspective. Handbook of Olfaction and Gustation, ed Doty RL (Marcel Dekker, New York), pp 275-294 33o. Turin, L., 2002. A method for the calculation of odor character from molecular structure. Journal of theoretical biology, 216(3), pp.367-385. 33p. Turin L.,, Yoshii F., (2003) Structure-odor relations: A modern perspective. Handbook of Olfaction and Gustation, ed Doty RL (Marcel Dekker, New York), pp 275-294 33q. Turin, L., 2007. The Secret of Scent: Adventures in Perfume and the Science of Smell, Harper Perennial edition, New York, 2007. 207 pp, Paperback, $13.95. ISBN 978-0-06-113384-8; Hettinger, T.P., Scent and Alchemy: The Paperback (A review of The Secret of Scent: Adventures in Perfume and the Science of Smell, by Luca Turin), Chemical Senses, Chem. Senses (2008) 33 (6): 575-579.; Turin, L., 2007. Letter to the Editor - Rebuttal to Hettinger's review of Turin's book (The Secret of Scent: Adventures in Perfume and the Science of Smell), Chem. Senses (2008) 33 (8): 683-684. 33r. Franco, M.I., Turin, L., Mershin, A. and Skoulakis, E.M., 2011. Molecular vibration-sensing component in Drosophila melanogaster olfaction. Proceedings of the National Academy of Sciences, 108(9), pp.3797-3802.; Hettinger, T.P., 2011. Olfaction is a chemical sense, not a spectral sense. Proceedings of the National Academy of Sciences, 108(31), pp.E349-E349.; Franco, M.I., Turin, L., Mershin, A. and Skoulakis, E.M., 2011. Reply to Hettinger: Olfaction is a physical and a chemical sense in Drosophila. Proceedings of the National Academy of Sciences, 108(31), pp.E350-E350 33s. Gane, S., Georganakis, D., Maniati, K., Vamvakias, M., Ragoussis, N., Skoulakis, E.M. and Turin, L., 2013. Molecular vibration-sensing component in human olfaction. PloS one, 8(1), p.e55780. 1-7. 33t. Block, Eric, Seogjoo Jang, Hiroaki Matsunami, Sivakumar Sekharan, Bérénice Dethier, Mehmed Z. Ertem, Sivaji Gundala et al. "Implausibility of the vibrational theory of olfaction." Proceedings of the National Academy of Sciences 112, no. 21 (2015): E2766-E2774.; Turin, L., Gane, S., Georganakis, D., Maniati, K. and Skoulakis, E.M., 2015. Plausibility of the vibrational theory of olfaction. Proceedings of the National Academy of Sciences, 112(25), pp.E3154-E3154.; Block, E., Jang, S., Matsunami, H., Batista, V.S. and Zhuang, H., 2015. Reply to Turin et al.: Vibrational theory of olfaction is implausible. Proceedings of the National Academy of Sciences, 112(25), pp.E3155-E3155. 33u. Vosshall, L.B., 2015. Laying a controversial smell theory to rest. Proceedings of the National Academy of Sciences, 112(21), pp.6525-6526. 33v. Triller, A., Boulden, E.A., Churchill, A., Hatt, H., Englund, J., Spehr, M. and Sell, C.S., 2008. Odorant–receptor interactions and odor percept: a chemical perspective. Chemistry & biodiversity, 5(6), pp.862-886. 33w. Polak, E.H., 1973. Multiple profile-multiple receptor site model for vertebrate olfaction. Journal of Theoretical Biology, 40(3), pp.469-484. 33x. Malnic, B., Hirono, J., Sato, T. and Buck, L.B., 1999. Combinatorial receptor codes for odors. Cell, 96(5), pp.713-723. 33y. Barwich, Ann-Sophie. "What is so special about smell? Olfaction as a model system in neurobiology." Postgraduate medical journal (2015): postgradmedj-2015. 34. Wood, W. B., et.al., Editors, Biochemistry A Problems Approach, , W. A. Benjamin, Inc., p. 195. 35. Zhao, H.; S. Firestein; C.A. Greer, NADPH-diaphorase localization in the olfactory system. Neuroreport., 6(1): 149-52 (1994) 36. Kuhl, P.W., A redox cycling model for the action of beta-adrenoceptor agonists., Experientia. 41: 1118-22 ( 1985) 37. Stemp, E.D. and J.K. Barton, Electron transfer between metal complexes bound to DNA: is DNA a wire?, Met. Ions Biol. Syst. 33:325-365 (1996) 37a. Wilson, E.K., DNA's conductance still confounds, Chem. Eng. News, July 27, 51-54 (1998); Porath, D., Bezryadin, A., De Vries, S. and Dekker, C., 2000. Direct measurement of electrical transport through DNA molecules. Nature, 403(6770), pp.635-638.; Giese, Bernd. "Long-distance charge transport in DNA: the hopping mechanism." Accounts of chemical research 33, no. 9 (2000): 631-636.; Astakhova, T. Yu, Vladimir Nikolaevich Likhachev, and George A. Vinogradov. "Long-range charge transfer in biopolymers." Russian Chemical Reviews 81, no. 11 (2012): 994-1010. 37b. Pace, U., Hanski, E., Salomon, Y. and Lancet, D., 1985. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature, 316(6025), pp.255-258.; Lancet, D., 1986. Vertebrate olfactory reception. Annual review of neuroscience, 9(1), pp.329-355.; Lancet, D. and Pace, U., 1987. The molecular basis of odor recognition. Trends in Biochemical Sciences, 12, pp.63-66.; Anholt, R.R., 1993. Molecular neurobiology of olfaction. Critical reviews in neurobiology, 7(1), pp.1-22. 37c. Henkin, R.I. and Velicu, I., 2008. cAMP and cGMP in nasal mucus: relationships to taste and smell dysfunction, gender and age. Clinical & Investigative Medicine, 31(2), pp.71-77.; Henkin, R.I., 2013. Nasal mucus contains cAMP and cGMP which are critical factors in preservation of normal olfaction. The FASEB Journal, 27(1_MeetingAbstracts), pp.1049. 38. Solms, J. Nonvolatile compounds and flavor, in Gustation and Olfaction, G. Ohloff and A. Thomas, Editors, Academic Press, 1971, pp.94-95. 39. Torii, K., R.H. Cagan., Biochemical studies of taste sensation. IX. Enhancement of L-[3H]glutamate binding to bovine taste papillae by 5'-ribonucleotides., Biochim. Biophys. Acta, Feb 7;627(3):313-323 (1980). 40. Kumazawa, T. and K. Kurihara, Large synergism between monosodium glutamate and 5'-nucleotides in canine taste nerve responses, Am. J. Physiol., Sep;259(3 Pt 2):R420-R426 (1990) 41. Ninomiya, Y., S. Kurenuma, T. Nomura, H. Uebayashi, H. Kawamura, Taste synergism between monosodium glutamate and 5'-ribonucleotide in mice, Comp. Biochem. Physiol. A;101(1):97-102 (1992) 42. Persaud, K.C., G.L. Heck, S.K. DeSimone, T.V. Getchell, J.A. DeSimone., Ion transport across the frog olfactory mucosa: the action of cyclic nucleotides on the basal and odorant-stimulated states. Biochim Biophys Acta, Sep 15;944(1):49-62 (1988). 43. Blaustein, D.N., R.B. Simmons, M.F. Burgess, C.D. Derby, M. Nishikawa, K.S. Olson, Ultrastructural localization of 5'AMP odorant receptor sites on the dendrites of olfactory receptor neurons of the spiny lobster. J Neurosci.; Jul;13(7):2821-2828 (1993) ....Page 1 on Olfaction....Page 2 on Olfaction... Page 3 on Olfaction....Next to Page 5 on Olfaction.....Home leffingwell@mindspring.com |
|
|
Copyright © Leffingwell & Associates
TERMS OF SERVICE.............PRIVACY POLICY